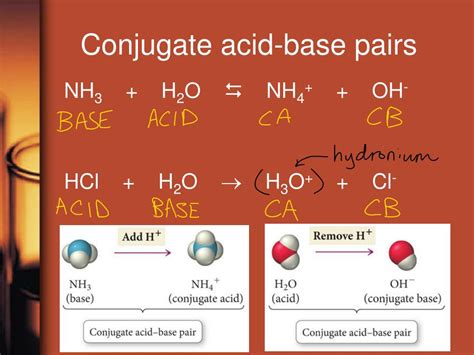
Which Of The Following Is A Conjugate Acid Base Pair. A) nh 4 + / nh 3. Similarly, hf is the conjugate acid of f.
2.7 Acids and Bases The BrønstedLowry Definition from qstion.co
Which of the following acids will have the strongest conjugate base? Nh3 and nh4+ o b. Write the formula of the conjugate acid.
2.7 Acids and Bases The BrønstedLowry Definition
Similarly, hf is the conjugate acid of f. A) nh 4 + / nh 3. H 2 0 is an acid and nh 3 is its conjugate base. Write the formula of the conjugate acid.
